

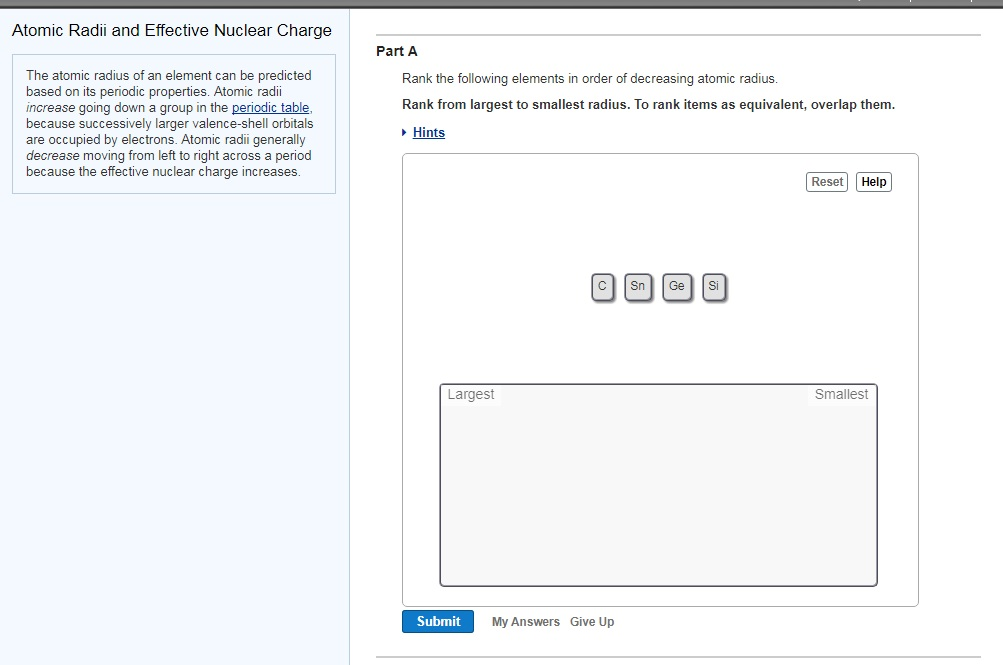
Practice Rank the following ions from largest to. Rank the following elements order of decreasing atomic radius Rank from largest to smallest radius. To rank items as equivalent, overlap them. When the radii of isoelectronic species are compared, as the nuclear charge increases, the size decreases. Rank from most to fewest valence electrons. Ar, P, Cl, Na, Al Part B Rank the following atoms by number of valence electrons. 6) Arrange the following elements in order of. To rank items as equivalent, overlap them. m) What is the special name given to the group in which these elements. Rank the following elements by increasing electronegativity: Sulfur, Oxygen, Neon, Aluminum. Rank from largest to smallest atomic radius. Terms in this set (14) Rank the following elements by increasing atomic radius: Carbon, Aluminum, Oxygen, Potassium. Rank the following elements in order of decreasing atomic radius. To rank items as equivalent, overlap them.

Rank from largest to smallest atomic radius. How fast is the radius of the balloon increasing. Find an expression for dr/dt, the rate at which the radius of the balloon is increasing. Rank the following elements in order of decreasing atomic radius. A spherical balloon is being inflated at a rate of 10 cubic centimeters per second. We illustrate the parameterization strategies, focusing on those combining experimental and atomistic simulation data, within the theoretical framework of multiscale approaches. Rank the following elements by atomic radius. Rank the following elements in order of decreasing atomic radius. Here, we review the latest advances in coarse-grained treatment of these systems, usually addressed using residue-level resolution for proteins and mesoscale for the nanoparticle. The use of multiscale approaches is inevitable because the adsorption events extend over a wide range of time and length scales, which require the system to be addressed at different resolution levels.
Theoretical modeling and simulations provide complementary approaches for experimental studies and are applied for exploring protein–particle surface-binding mechanisms, the determinants of binding specificity toward different surfaces, and the thermodynamics and kinetics of adsorption. Understanding protein interactions with inorganic nanoparticle is central to the rational design of new tools in biomaterial sciences, nanobiotechnology, and nanomedicine.


 0 kommentar(er)
0 kommentar(er)
